The FDA updated its mammogram guidelines Thursday to require patients get information about breast density, which is a risk factor for cancer.
People across the United States receiving mammograms will soon be given information about their breast density – a trait that can make cancer harder to detect.
The U.S. Food and Drug Administration published updates Thursday that would require facilities to “notify patients about the density of their breasts,” among additional oversight of mammography facilities and other changes.
This means people with dense breasts will be notified that their status “makes it harder to find breast cancer.” Those patients will be advised to speak with medical providers about the results.
“Today’s action represents the agency’s broader commitment to support innovation to prevent, detect and treat cancer,” said Dr. Hilary Marston, the FDA’s chief medical officer.
Providers will have 18 months to comply with the regulations, though some states already require women receive information about breast density.
Here’s what you need to know.
What is a mammogram?
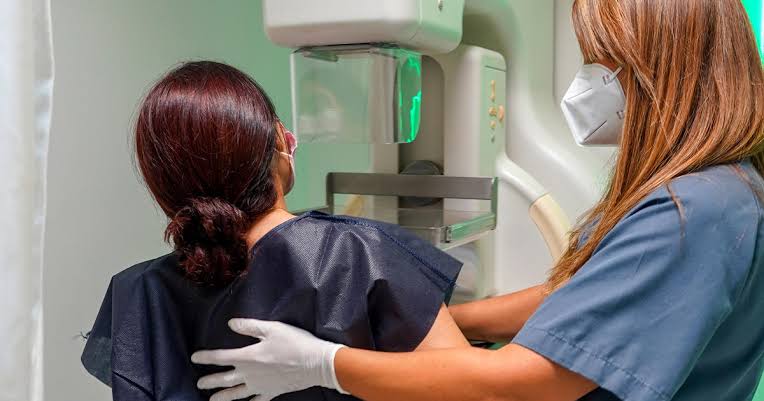
A mammogram is an X-ray of a person’s breasts. The imaging, which typically takes less than 30 minutes, is used for breast cancer screening, according to the Mayo Clinic. They can also be used to examine changes in a person’s breasts, such as a new lump, pain and more.
During a mammogram, a person’s breasts “are compressed between two firm surfaces to spread out the breast tissue,” according to the Mayo Clinic. Next, an X-ray captures images of the tissue that are “examined for signs of cancer.”
Dr. Lauren Friedlander, radiologist at NewYork-Presbyterian Westchester and associate professor of radiology at Columbia University Irving Medical Center, called mammography “the mainstay of breast cancer screening.”
“There are certainly many tests that we have to screen for breast cancer, and many newer tests, but mammography is really the only test that we have that has a proven mortality benefit that’s been studied for many, many years,” she said.
Mammograms can also detect breast cancer before a person might experience symptoms.
What is the FDA changing about mammograms?
Mammogram X-rays won’t change under the FDA’s updates. Instead, the requirements seek to standardize the information given to people following the scans.
Under the update, people with dense breasts will be notified of the status and be urged to speak with a medical provider about it. Federal guidelines don’t require next steps for these people, but their doctors might recommend additional scans, such as an ultrasound or MRI.
Friedlander called the FDA’s announcement “a national standard for patient reporting.”
“In addition to receiving a notification that their results are benign, or negative, patients will receive notification that they have dense breast tissue or that they do not have dense breast tissue,” she explained. “There’s language in the requirements that lets patients know that women who do have dense breast tissue may benefit from additional screening imaging exams.”
“That’s important because it really promotes a conversation between a patient and her physician in order to make good decisions about what other things may be done to help a woman reduce her risk of breast cancer,” she said.
What are dense breasts?
Breast density “reflects the amount of fibrous and glandular tissue in a woman’s breasts compared with the amount of fatty tissue in the breasts,” according to the U.S. Centers for Disease Control and Prevention. Connective and glandular tissue is white on X-rays – the same color as a growth in the breast. That makes it potentially harder for medical professionals to read mammograms.
About half of women over 40 in the U.S. have dense breast tissue, according to the FDA. This tissue can make it harder to detect cancers, and dense breasts have also been “identified as a risk factor for developing breast cancer.”
“Dense breast tissue is not something that can be identified on physical exam, either by a physician or by a patient examining herself,” Friedlander said. “It’s really based upon the appearance of the breasts on imaging and specifically on mammography.”
At what age should you get a mammogram?
The United States Preventive Services Task Force, which is a group of doctors and health experts who can make recommendations for medical professionals, says women 50 to 74 years old and at average risk for breast cancer should get a mammogram every two years.
Women between the ages of 40 and 49 should consult with their doctor about when to have a mammogram and how often they may want to schedule one.
The American Cancer Society’s screening recommendations say women ages 40 to 44 should have the choice to begin screening with a mammogram annually. The organization recommends that women ages 45 to 54 should get mammograms annually.
But women with a family history of breast cancer may want to start screening “10 years earlier than the first affected relative in the family,” according to the Cleveland Clinic.
USA TODAY






